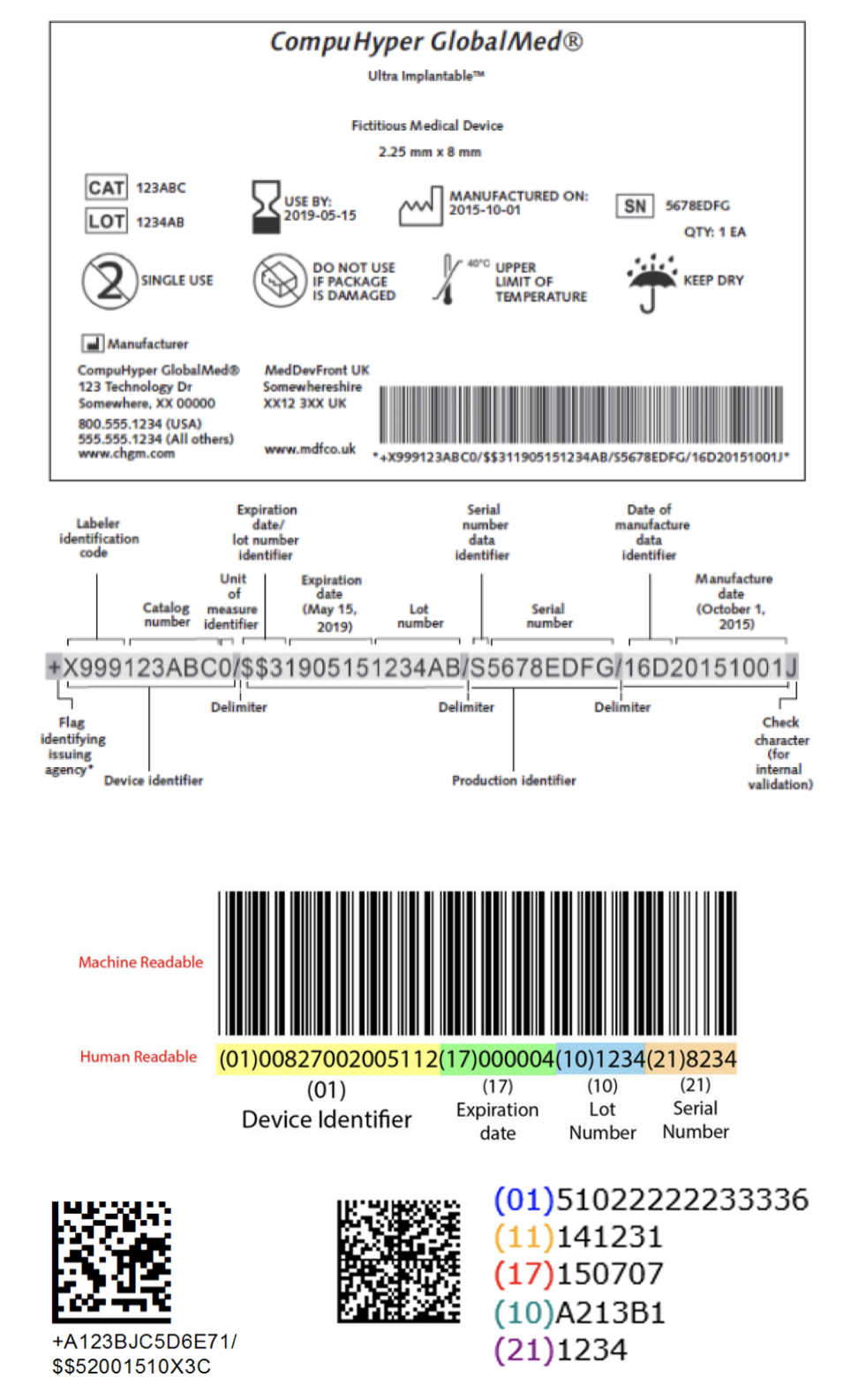
A unique device identifier (UDI) is a unique code that is required on the label and packaging of a medical device in both human and machine-readable forms.
A UDI is also required on devices that are intended for more than one use and that are reprocessed before each use.
The UDI consists of two parts:
- the Device Identifier (DI) identifies the manufacturer and model of the device
- the Production Identifier (PI) identifies, as available for the device, the lot number, serial number, expiration date, and/or date of manufacture
The 2013 Unique Device Identification System Rule mandated manufacturers to assign unique device identifiers to their marketed devices and to submit required device identification information to the FDA’s Global Unique Device Identification Database (GUDID).
Most high-risk (Class III, such as coronary stents) and moderate-risk (Class II, such as GI endoscopes, knee implants) have UDIs. This includes implantable devices.
Some Class I devices also have UDIs.
Detailed information on UDI including the Timeline for manufacturers is available through the U.S. Food and Drug Administration Unique Device Identification System (UDI System).
Example UDI Formats
What is GUDID?
The Global Unique Device Identification Database (GUDID) is a database containing device identification information and device characteristics submitted to the US FDA for all devices with UDIs.
This information is publicly accessible through AccessGUDID.
Hospitals, clinicians, patients, researchers, and all interested parties can download information on specific medical devices.
Detailed information on GUDID is available through the U.S. Food and Drug Administration Global Unique Device Identification Database (GUDID) and AccessGUDID.
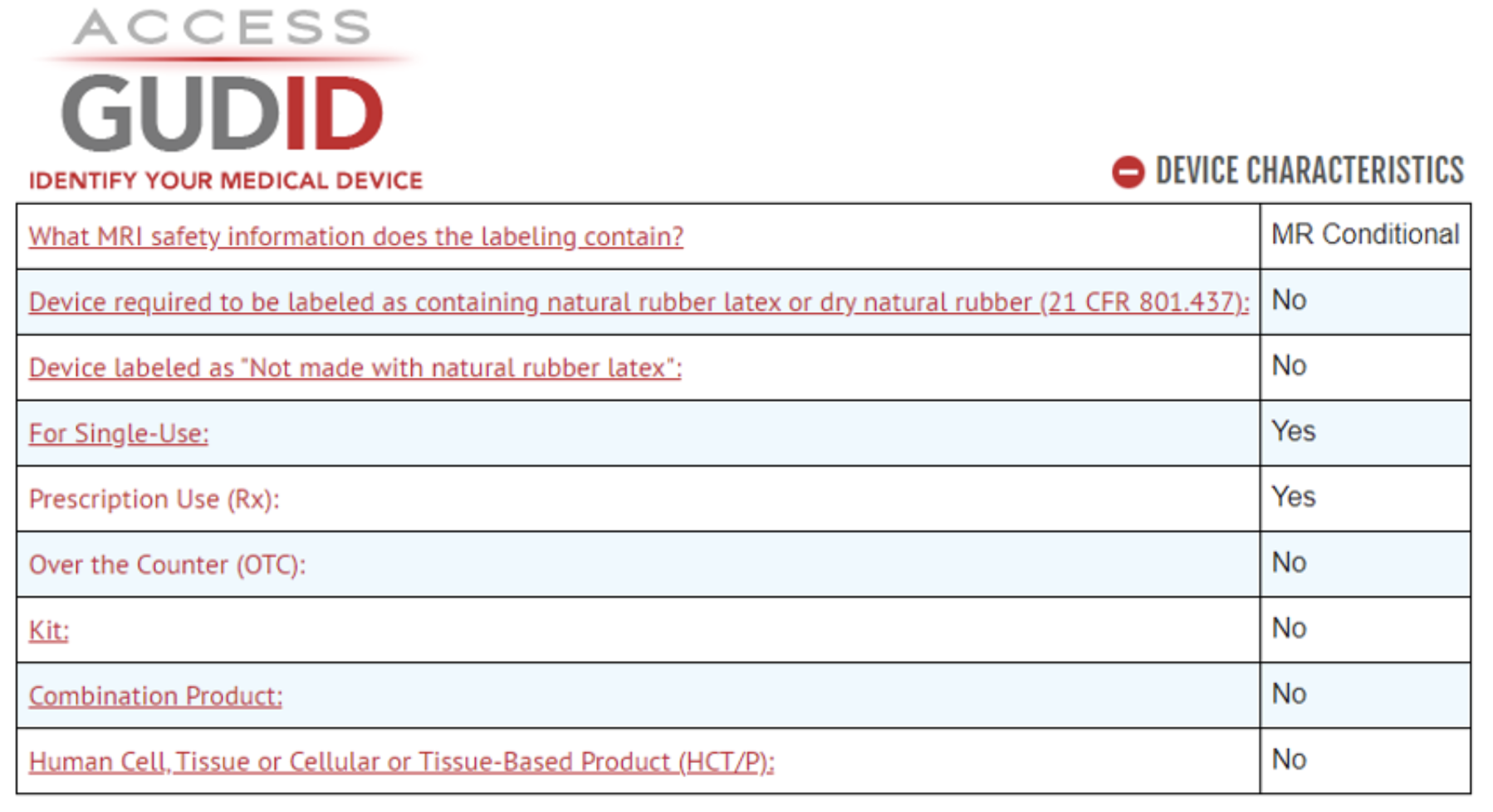
Example of Device Characteristics
Benefits of UDI
A UDI is a medical device identification standard that can be electronically captured and documented in health IT systems and transmitted as needed. It can be used for clinical, safety surveillance, research, regulatory, and operational purposes.
The capability of a comprehensive UDI-enabled system in health care that routinely uses UDIs is significant. Supported is patient safety, quality of care, efficiency, cost-savings, vigilance, and innovation. However, before these can be broadly supported, UDI adoption and use across health care is necessary.
CLINICAL
When a device is used in in patient care, electronic capture and documentation of the UDI in the electronic health record and other health IT systems electronically links that device to the patient. This provides capabilities to support clinical decision-making, quality of care and safety for that patient in the short and long term.
UDI enables:
- Standardized documentation of devices used in patient care
- Ease of device identification during a patient’s care
- Prior to a revision procedure or other surgery
- Prior to scheduling an MRI
- In an emergency
- Accuracy and efficiency in a device recall
- Advances in post-market surveillance, research and device evaluations to inform clinical decision-making, support patient safety, and improve quality of care
PATIENT INFORMATION
Once UDIs for devices used in a patient care are electronically documented, the UDIs can be shared with patients in their patient portal, on an implant card or through other methods.
UDI enables:
- Standard identifying information for patients on devices used in their care, including implanted devices
- Patient sharing of standard identifying information on their devices with clinicians involved in their care
- Furthered patient engagement and communication about the devices used in their medical care
SAFETY SURVEILLANCE, RESEARCH
Once UDIs for devices used in clinical care are electronically documented, this standard is available for transmission and use in device reports and datasets.
Electronic health records, administrative claims, registries and other datasets contain valuable data that can be used to assess device safety and performance, effectiveness, comparative effectiveness, and differences in patient sub-populations. This supports the generation of meaningful real-world evidence that can strengthen postmarket surveillance of medical devices.
UDI enables:
- Standard device documentation for FDA, registries, coordinated registry networks (CRNs), NESTcc, researchers, manufacturers, other data networks and stakeholders
- Precise identification of devices and record accuracy in adverse event reports as well as greater ability to obtain the counts of adverse events
- A reliable standard in FDA and manufacturer safety alerts and recall communications
- Augmentation of tracking device performance and research by clinical registries and manufacturers
- Linkage of clinical and device data for device safety surveillance and effectiveness research
OPERATIONS
When UDIs are used in the health care supply chain, a standard is available to identify devices through distribution and use and for data exchange between trading partners. This encompasses device movement from manufacturers, through distribution, into health systems and for clinical use.
UDI enables:
- Standard device documentation for use in inventory management, implant tracking, recall and expired device management
- A reliable standard in contracting, purchasing, and charge capture
- Linkage of data for use in outcomes analyses
- Advances in master data management and supply chain modernization
More information is available through U.S. Food and Drug Administration Benefits of a UDI System.
National Policy and Initiatives
Policy Timeline
Supporting Initiatives
Learning UDI Community (LUC)
A multi-stakeholder coalition working to develop a common understanding and approach for UDI adoption in health care settings. Expert workgroups address community-identified UDI adoption issues and develop tools, resources, best practices, and implementation guidelines.
Medical Device Epidemiology Network (MDEpiNet)
A public-private partnership focused on innovative data source development and analytic methodologies to enhance regulatory science applied to medical device research and surveillance.
National Evaluation System for health Technology (NEST)
NESTcc is a coordinating center for a network of voluntary research partners working to advance the generation and use of real-world evidence for medical devices as well as a collaborative community focused on thought leadership and innovation in the medical device ecosystem.
Initiative Spotlight: Evaluation of Uptake of UDIs by Health Systems
INVESTIGATORS

Sanket Dhruva, M.D., MHS

Joseph Drozda, Jr., M.D.

Jennifer Ridgeway, Ph.D.

Joseph Ross, M.D., MHS

Natalia Wilson, M.D., MPH
UDIs in Databases
Research Project Databases with UDIs
Building UDI into Longitudinal Data for Medical Device Evaluation (BUILD)
Publications and Links
Commentaries on UDI Value
- Krupka DC, Wilson NA, Reich AJ, Weissman JS. The Post-Market Surveillance System for Implanted Devices is Broken. Here’s How CMS and The FDA Can Act Now to Fix It. Health Affairs Blog, April 23, 2021. DOI: 1377/forefront.20210420.717948
- Kinard M, McGiffert L. Medical Device Tracking—How It Is and How It Should Be. JAMA Intern Med. 2021;181(3):305-306. DOI: 1001/jamainternmed.2020.7797
- Tomes M. Identification and Market Removal of Risky Medical Devices. JAMA Intern Med. 2020;180(11):1426-1427. DOI: 1001/jamainternmed.2020.3512
- Aston JW, Howarth AL, Wilson N, Mahabir RC. The Value of Unique Device Identification in Plastic Surgery. Aesthet Surg J. 2018; 38(11): 1264-1266. DOI: 10.1093/asj/sjy210
- Resnic FS, Matheny ME. Medical Devices in the Real World. N Engl J Med. 2018; 378(7):595-597. DOI: 10.1056/NEJMp1712001
- Dhruva SS, Ross JS, Schulz WL, Krumholz HM. Fulfilling the Promise of Unique Device Identifiers. Ann Intern Med. 2018;169(3):183-185. DOI: 7326/M18-0526
- Kramer DB, Yeh RW. Practical Improvements for Medical Device Evaluation. JAMA. 2017;318(4):332-334. DOI: 10.1001/jama.2017.8976
- Shuren J, Califf RM. Need for a National Evaluation System for Health Technology. 2016; 316(11): 1153-1154. DOI: 10.1001/jama.2016.8708
- Moscovitch B, Rising J, Daniel G, Drozda J. Time to Fix The Black Hole in Medicare Data. Health Affairs Blog. June 29, 2016. 1377/forefront.20160629.055612
- Graham J, Cooper S, Mackeen D. How safety concerns about Essure reveal a path to better device tracking, Health Affairs Blog, October 15, 2015. 1377/forefront.20151015.051219
- Rising J, Moscovitch B. The Food and Drug Administration’s Unique Device Identification System Better Postmarket Data on the Safety and Effectiveness of Medical Devices. JAMA. 2014; 174(11): 1719-20. DOI:1001/jamainternmed.2014.4195
- Wilson NA, Drozda J. Value of Unique Device Identification in the Digital Health Infrastructure. JAMA. 2013;309(20):2107-2108. DOI: 10.1001/jama.2013.5514
- Gross TP, Crowley J. Unique Device Identification in the Service of Public Health. N Engl J Med. 2012;367(17):1583-1585. DOI: 10.1056/NEJMp1113608
- McCullough C, Reed T, Kaufman-Rivi D. A Tool to analyze Medical Device Problems: FDA Event Problem Codes. Journal of Clinical Engineering; 2012;37:56-62. DOI: 10.1097/JCE.0b013e31824c99f1
- Reed T, Kaufman-Rivi D. FDA Adverse Event Problem Codes: Standardizing and Classification of Device and Patient Problems Associated with Medical Device Use. Biomed Instrum Technol 2010; 44: 248-56. DOI: 10.2345/0899-8205-44.3.248.
UDI Implementation in Health Systems
- Wilson NA, Tcheng JE, Graham J, Drozda JP Jr. Advancing Patient Safety Surrounding Medical Devices: Barriers, Strategies, and Next Steps in Health System Implementation of Unique Device Identifiers. Med Devices (Auckl). 2022;15:177-186. https://doi.org/10.2147/MDER.S364539
- Tcheng JE, Nguyen MV, Brann HW et al. The Medical Device Unique Device Identifier as the Single Source of Truth in Healthcare Enterprises – Roadmap for Implementation of the Clinically Integrated Supply Chain. Med Devices (Auckl). 2021;14:459-467. DOI: 2147/MDER.S344132
- Wilson NA, Tcheng JE, Graham J, Drozda JP Jr. Advancing Patient Safety Surrounding Medical Devices: A Health System Roadmap to Implement Unique Device Identification at the Point of Care. Med Devices (Auckl).2021;14:411-421 https://doi.org/10.2147/MDER.S339232
- Wilson N, Jehn M, Kisana H, et al. Nurses’ Perceptions of Implant Barcode Scanning in Surgical Services. Comput Inform Nurs. 2020;38(3):131-138. DOI: 1097/CIN.0000000000000579
- Wilson NA. Building UDI into Longitudinal Data for Medical Device Evaluation (BUILD) Point of Care Capture of UDI for Implantable Devices Final Summary Report & Roadmap U.S. Food and Drug Administration. 2019.
- Drozda JP, Roach J, Forsyth T, Helmering P, Dummitt B, Tcheng JE. Constructing the informatics and information technology foundations of a medical device evaluation system: a report from the FDA unique device identifier demonstration. JAMIA 2018; 25(2):111-120. DOI: 1093/jamia/ocx041
- Drozda JP, Dudley C, Helmering P, Roach J, Hutchison L. The mercy unique device identifier demonstration project: Implementing point of use product identification in the cardiac catheterization laboratories of a regional health system. Healthcare. 2016;4(2): 116-119. DOI: 10.1016/j.hjdsi.2015.07.002
- The Brookings Institution. Unique Device Identifiers (UDIs): A Roadmap for Effective Implementation. December 2014.
- Campion TR, Johnson SB, Paxton EW, Mushlin AI, Sedrakyan A. Implementing Unique Device Identification in Electronic Health Record Systems. Organizational, Workflow, and Technologic Challenges. Med Care. 2014;52(1):26-31. DOI: 1097/MLR.0000000000000012
- Drozda, JP, Helmering P, Moore V, Smith TR. UDI Demonstration Project Final Report. December 2013.
UDI Use in Patient Care
- Wilson NA, Reich AJ, Graham J, Bhatt DL, Nguyen LL, Weissman JS. Patient perspectives on the need for implanted device information: Implications for a post-procedural communication framework. Health Expect. 2021;00:1–12. DOI: 10.1111/hex.13273
- Wilson N, Broatch J, Jehn M, Davis CM. National projections of time, cost and failure in implantable device identification: Consideration of unique device identification use. Healthcare. 2015;3(4):196-201. DOI: 1016/j.hjdsi.2015.04.003
- Wilson NA, Jehn M, York S, Davis CM. Revision Total Hip and Knee Arthroplasty Implant Identification: Implications for Use of Unique Device Identification 2012 AAHKS Member Survey Results. J Arthroplasty. 2014;29:251-255. DOI: 1016/j.arth.2013.06.027
UDI Use in Claims
- Krupka DC, Graham J, Wilson NA, et al. Transmitting Device Identifiers of Implants from the Point of Care to Insurance Claims: A Demonstration Project. J Patient Saf. 2021;17(3):223-230. DOI:10.1097/PTS.0000000000000828
- Frontz AJ. Hospitals Did Not Comply with Medicare Requirements for Reporting Cardiac Device Credits. Office of Public Affairs, Dept. HHS. 2020 November; Report No. A-01-18-00502
- Zerhouni YA, Krupka DC, Graham J, et al. UDI2Claims: Planning a Pilot Project to Transmit Identifiers for Implanted Devices to the Insurance Claim.J Patient Saf. 2018. DOI: 1097/PTS.0000000000000543
- Jarmon GL. Hospitals Did Not Comply with Medicare Requirements for Reporting Certain Cardiac Device Credits. Office of Public Affairs, Dept. HHS. 2018 March. Report No. A-05-16-0059
- Levinson DR. Shortcomings of Device Claims Data Complicate and Potentially Increase Medicare Costs for Recalled and Prematurely Failed Devices. Office of Public Affairs, Dept. HHS. 2017 September; Report No. A-01-15-00504
- Harvard Medical School/Brigham and Women’s Hospital/Geisinger Health System Pilot Study. Transmitting the UDI from the Point of Use to Insurance Claims: Changes in Workflows and Information Systems. May 2017
UDI Use in Registries
- Jones WS, Krucoff MW, Morales P, et al. Registry Assessment of Peripheral Interventional Devices (RAPID): Registry assessment of peripheral interventional devices core data elements. J Vasc Surg. 2018;67(2):637-644. DOI: 1016/j.jvs.2017.07.141
UDI in Real-World Data for Research on Medical Device Safety and Effectiveness
- Drozda JP Jr, Graham J, Muhlestein JB, et al. Multi-institutional distributed data networks for real-world evidence about medical devices: building unique device identifiers into longitudinal data (BUILD). JAMIA Open. 2022;5(2):1-11. https://doi.org/10.1093/jamiaopen/ooac035
- Timbie JW, Kim AY, Concannon TW. Use of Real-World Evidence for Regulatory Approval and Coverage of Medical Devices: A Landscape Assessment. Value Health. 2021; 24(12):1792–1798. DOI: https://doi.org/10.1016/j.jval.2021.07.003
- Jiang G, Dhruva SS, Chen J et al. Feasibility of capturing real-world data from health information technology systems at multiple centers to assess cardiac ablation device outcomes: A Fit-for-purpose informatics analysis report. JAMIA. 2021;28(10):2241-2250. DOI: 10.1093/jamia/ocab117
- Tcheng JE, Fleurence R, Sedrakyan A. Electronic health data quality maturity model for medical device evaluations. BMJ Surg Interv Health Technologies 2020;2:e000043. DOI:10.1136/bmjsit-2020-000043
- Drozda J, Zeringue A, Dummitt B, Yount B, Resnic F. How real-world evidence can really deliver: a case study of data source development and use. BMJ Surg Interv Health Technologies. 2020;2:e000024. DOI:10.1136/bmjsit-2019-000024
- Reed TL, Drozda JP, Baskin KM, et al. Advancing medical device innovation through collaboration and coordination of structured data capture pilots: Report from the Medical Device Epidemiology Network (MDEpiNet) Specific, Measurable, Achievable, Results-Oriented, Time Bound (SMART) Think Tank. Healthcare. 2017;(4): 158-164. DOI: 10.1016/j.hjdsi.2016.10.004
- Tcheng JE, Crowley J, Tomes M, et al. Unique device identifiers for coronary stent postmarket surveillance and research: A report from the Food and Drug Administration Medical Device Epidemiology Network Unique Device Identifier Demonstration. Am Heart J. 2014;168(4):405-413. DOI: 1016/j.ahj.2014.07.001
Reports
- World Health Organization. Executive Board Report. Standardization of medical devices nomenclature. International classification, coding and nomenclature of medical devices. Report by the Director-General. 150/14 Add 1. Jan 2022.
- AHRMM Learning UDI Community. Unique Device Identifier (UDI) Impacts on Recall Management Workgroup Report. 2021.
- Timble JW, Concannon TW, Kim AY, Baker L, Gahlon G, Li R. Using Real-World Evidence to Support Regulatory Decision making for Medical Devices. Interim Report on Lessons from the National Evaluation System for health Technology Coordinating Center (NESTcc) Test-Cases. RAND Corporation. 2021. https://nestcc.org/independent-assessment-of-the-nestcc-test-cases-rand-interim-report/
- Augmented Unique Device Identifier (AUDI) Workgroup Report
- Food and Drug Administration. Office of Medical Products and Tobacco. Center for Devices and Radiological Health: Medical Device Safety Action Plan: Protecting Patients, Promoting Public Health. A CDRH Report.
- AHRMM Learning UDI Community. The Business Case for the UDI Work Group Report. 2018.
- Duke-Margolis Center for Health Policy. The National Evaluation System for health Technology (NEST): Priorities for Effective Early Implementation. A NEST Planning Board Report. September 2016.
- Duke-Margolis Center for Health Policy. Better Evidence on Medical Devices: A Coordinating Center for a 21st Century National Medical Device Evaluation System. April 2016.
- Pew Charitable Trusts. Implementing Unique Device Identification. September 2015.
- The Brookings Institution. Strengthening Patient Care: Building an Effective National Medical Device Surveillance System. February 2015.
- Center for Devices and Radiological Health. U.S. Food and Drug Administration. Strengthening Our National System for Medical Device Postmarket Surveillance. Update and Next Steps. April 2013.
- Center for Devices and Radiological Health. U.S. Food and Drug Administration. Strengthening Our National System for Medical Device Postmarket Surveillance. September 2012.
Links
- AccessGUDID
- Building UDI into Longitudinal Data for Medical Device Evaluation (BUILD)
- FDA Benefits of a UDI System
- FDA Global Unique Device Identification Database (GUDID)
- FDA Timeline for Manufacturers
- FDA Unique Device Identification System (UDI System)
- International Medical Device Regulators Forum (IMDRF)
- Learning UDI Community (LUC)
- Medical Device Epidemiology Network (MDEpiNet)
- National Evaluation System for health Technology (NEST)
- ONC requirement for an implantable device list for Electronic Health Record (EHR) Certification
- ONC UDI in the Common Clinical Data Set (CCDS)
- UDI2Claims
- Unique Device Identification System Rule
- UDI Issuing Agencies
- X12 formally recommends inclusion of the DI for high risk implantable devices in the insurance claim form
- X12 response to Senators Warren & Grassley request for DI in claims update
Contacts
Sanket Dhruva, MD, MHS
UCSF School of Medicine
Sanket.Dhruva@ucsf.edu
Natalia Wilson, MD, MPH
Arizona State University
Natalia.wilson@asu.edu