December 16, 2020
The objective of this Test-Case was to develop objective performance criteria (OPC) or goals (OPG) for major outcomes following (Class II or III) implantable device use in primary hip and knee replacements. An OPC is a target value derived from historical data from clinical studies and/or registries which may be used for comparison of safety or effectiveness endpoints for medical devices.
The study explored the feasibility of using several real-world data (RWD) sources scientifically to develop OPC that could be utilized generally in pre-market device regulation of new devices, pre-market investigational device exemption (IDE) clinical studies, and to support label change decisions for existing devices.
Several experts involved in this study shared their insight at the NEST Forum. Below are some key findings from the discussion:
Objective Performance Criteria (OPC) has the potential to increase accessibility to innovative devices.
Hip and knee joint replacements are the most common medical procedures in the U.S., and there has been a continuous increase in the use of these two procedures over time.1 The major outcome measures following hip and knee replacements are revisions and quality of life (QoL) changes. However, specific objective measures for outcomes after treatment have not been developed by the healthcare community.
Mr. Paul Voorhorst, Vice President of Worldwide Clinical Research at DePuy Synthes Companies, discussed the value OPC adoption would bring to the surgical arena. “I think this is an area ripe for OPCs because performing randomized controlled trials in surgical treatments can be very difficult for numerous reasons well-published in literature. Adoption of pre-market OPC designs could improve the timely access to innovative devices.”
This Test-Case used real-world evidence (RWE) in registries and claims data to explore OPC measures.
Dr. Art Sedrakyan, professor at Weill Cornell Medicine and Director of Medical Device Epidemiology Network’s (MDEpiNet) Coordinating Center, outlined the goals of this Test-Case and the data sources used in his presentation. “We had two goals; first, to develop performance criteria for all cause and cause-specific revisions for two years following primary hip and knee replacements. We used data from Kaiser Permanente and FORCE-TJR registry. We used claims data from the states of New York and California. We also performed a systematic analysis of literature by extracting data from studies conducted in the U.S. over the past ten years. The second goal was to use and develop objective performance criteria for patient-reported outcome measures at two years using the FORCE-TJR registry and literature data.”
Dr. Elizabeth Paxton, Director of Surgical Outcomes at Kaiser Permanente, presented on the registry evaluations for safety and effectiveness. Dr. Paxton noted, “For total hip replacements for all patients, the overall two-year revision rate was 2.1%. We also looked at age and gender; females had a slightly higher rate of revision, which was predominant in the younger age group.”
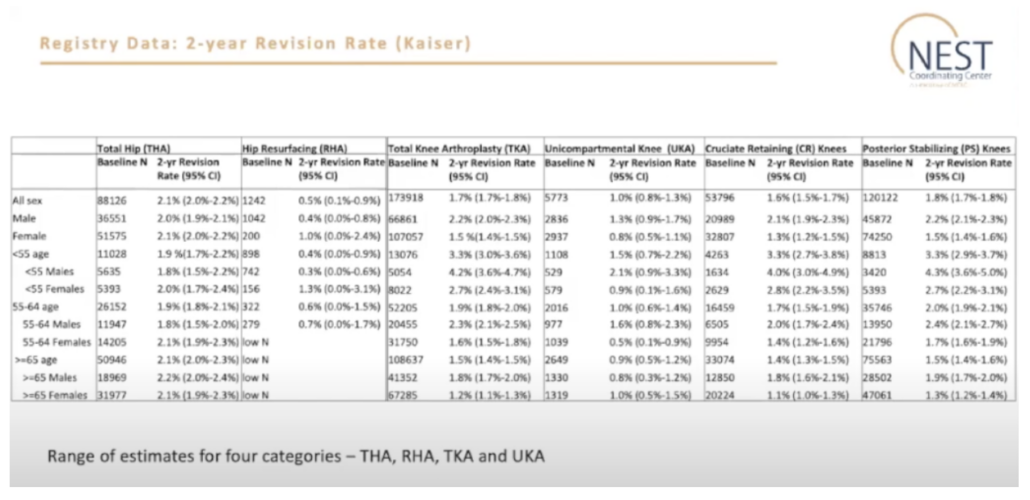
Figure 1 – Registry Data: Two-Year Revision Rate (Kaiser)
NESTcc fosters engagement between experts from diverse areas of study, which helps to demonstrate the impact of RWE on the device total product lifecycle (TPLC).
Dr. Sedrakyan summarized the findings of the study and highlighted the importance of collaboration with the U.S. Food and Drug Administration (FDA). “The use of OPCs can help us advance safety and effectiveness of implants throughout the TPLC. We believe that collaborating with the FDA helped us to advance these OPCs for cardiovascular neurology device settings. Our work can help advance the regulatory and stakeholder overall decision-making in the area of orthopedic devices.” This study can help advance the adoption of a less burdensome approach for evidence generation, bringing benefits to stakeholders in terms of cost and efficiency.
Thank you to the partners and experts who joined us to discuss this Test-Case. We look forward to our continued conversation and collaboration on issues of pressing importance to the medical device ecosystem.
The NEST Forum shared insights and perspectives on NEST’s use of real-world data to move safety and innovation forward and help change the paradigm of clinical research. We are grateful for the medical device ecosystem leaders who joined us and look forward to continued conversation and collaboration on issues of pressing importance to health care.
All session recordings are available on the NEST Forum event page.

